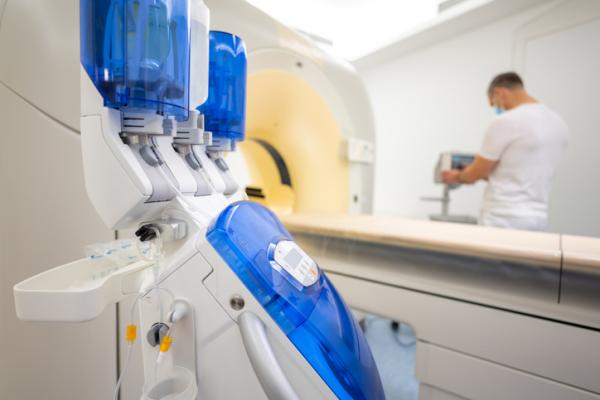
FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.

Bracco Diagnostics 00270131530 - McKesson Medical-Surgical
Contrast Media Injectors

Siemens Healthineers Announces FDA Clearance of ARTIS icono ceiling Angiography System
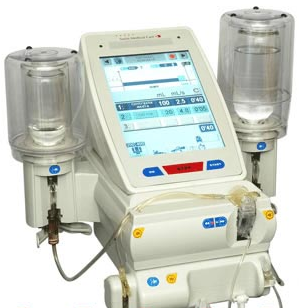
Bracco Acquires Contrast Injector Maker Swiss Medical Care

Generic, FDA approved contrast agent set to hit the market in wake of nationwide shortage

FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages
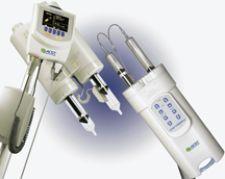
ACIST Features Contrast Injectors, Software

FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages
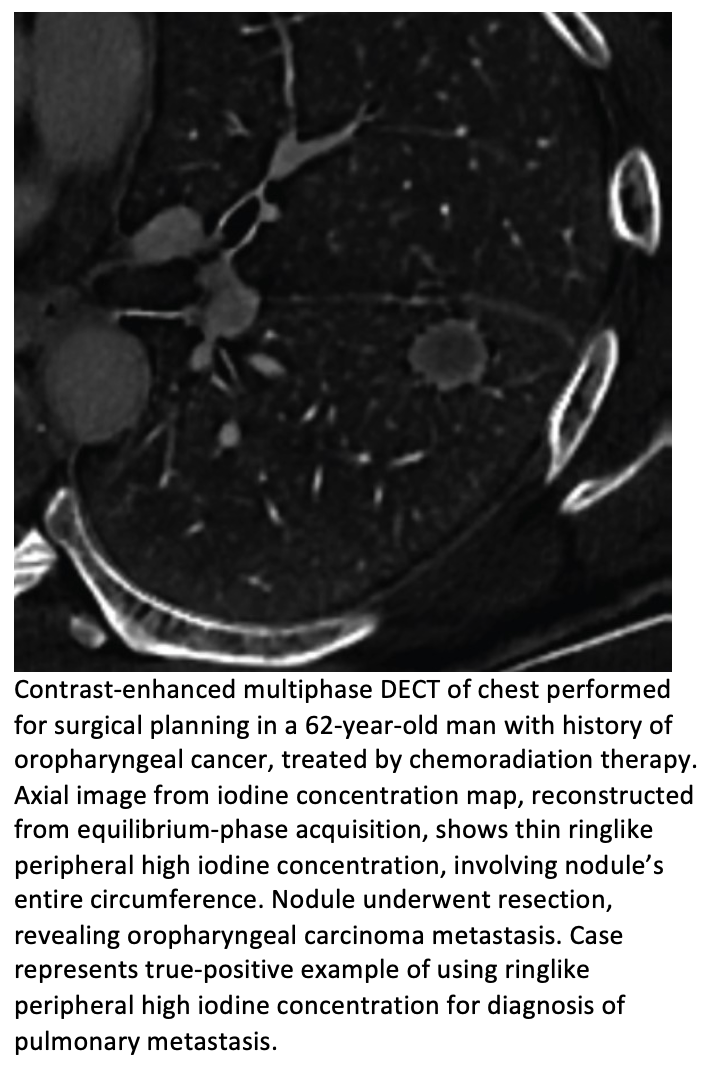
articles • APPLIED RADIOLOGY

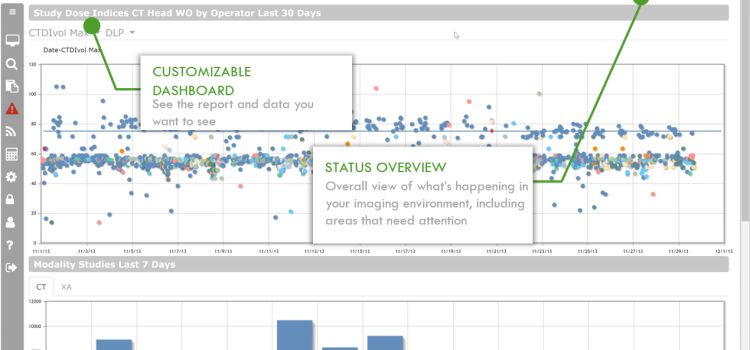
SEC Filing GE HealthCare
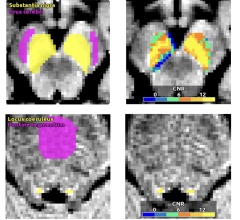
Guerbet Announces ACR Committee on Drugs and Contrast Media Classifies Elucirem (Gadopiclenol) Injection a Group II Agent
Angiography Imaging Technology News - 阿根廷vs乌拉圭直播
Contrast Media Injectors

gehc-20221231