14.6: Buffers - Chemistry LibreTexts
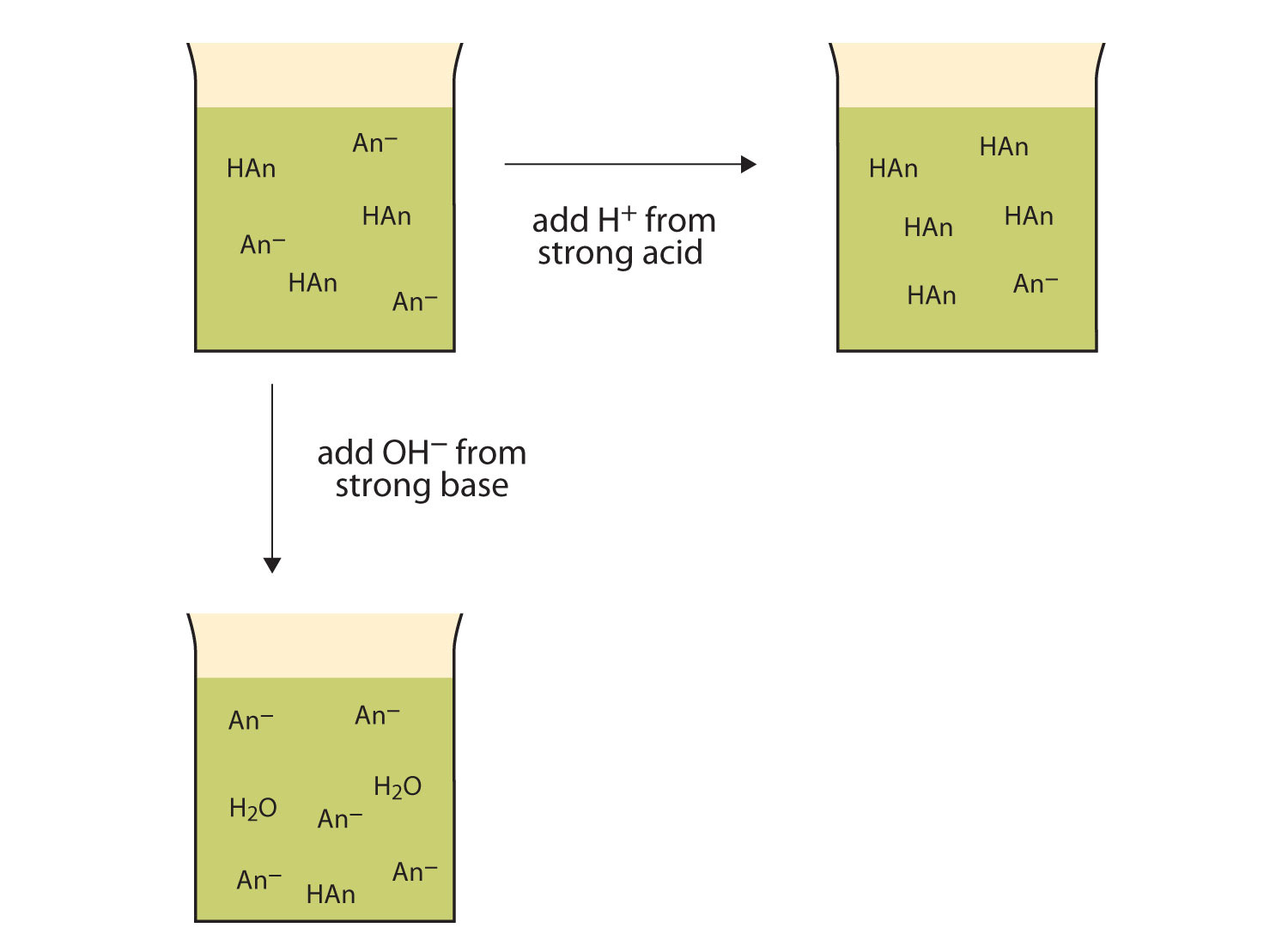
A solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Unlike in the case of an acid, base, or salt solution, the …
A solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Unlike in the case of an acid, base, or salt solution, the hydronium ion concentration of a buffer solution does not change greatly when a small amount of acid or base is added to the buffer solution. The base (or acid) in the buffer reacts with the added acid (or base).

5.1: Day 36- Buffer Solutions - Chemistry LibreTexts

Chapter 16.6: Buffers - Chemistry LibreTexts
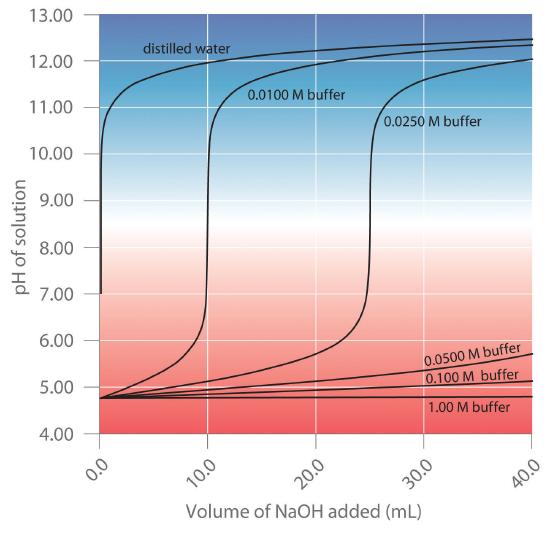
Chapter 16.6: Buffers - Chemistry LibreTexts
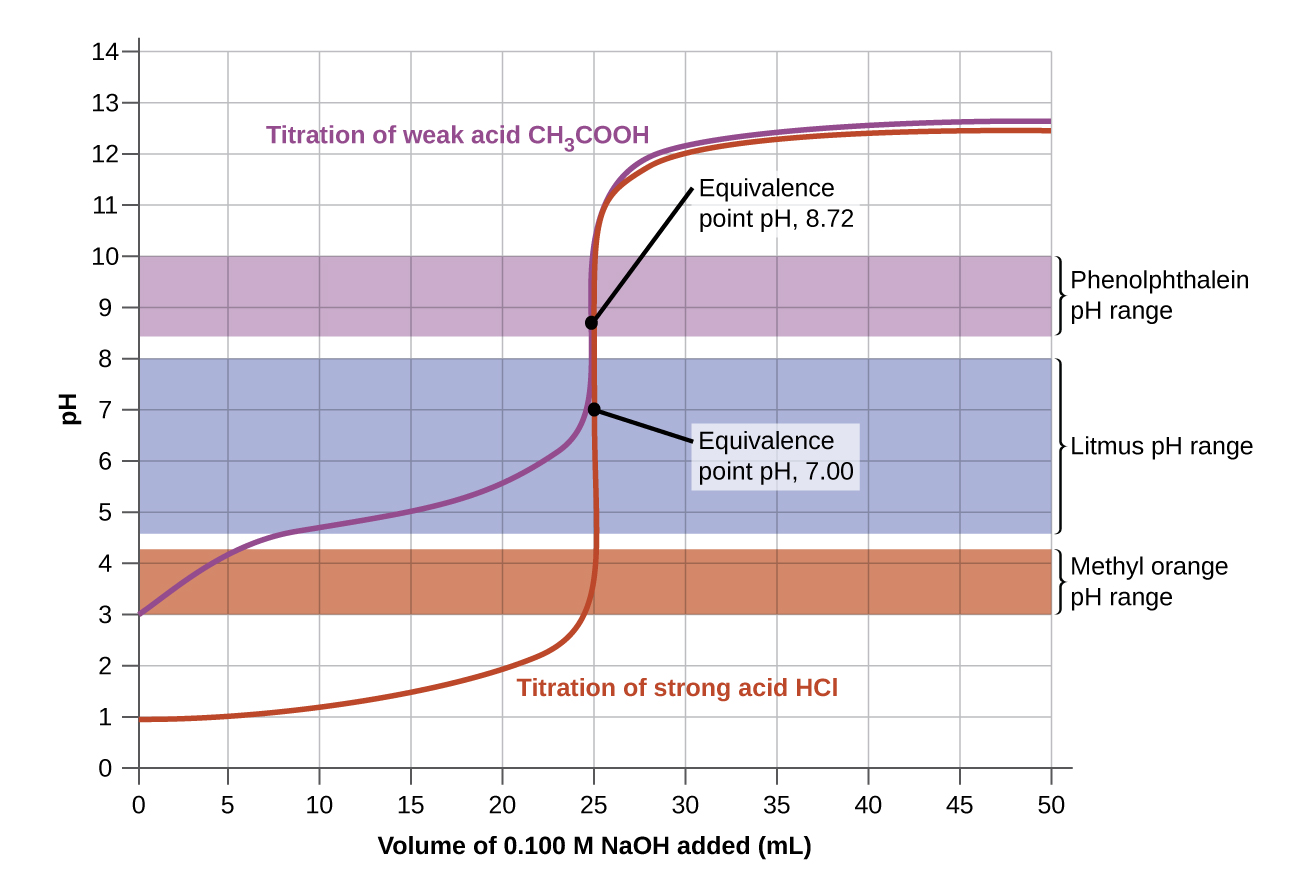
14.7: Acid-Base Titrations - Chemistry LibreTexts

Chapter 16.6: Buffers - Chemistry LibreTexts
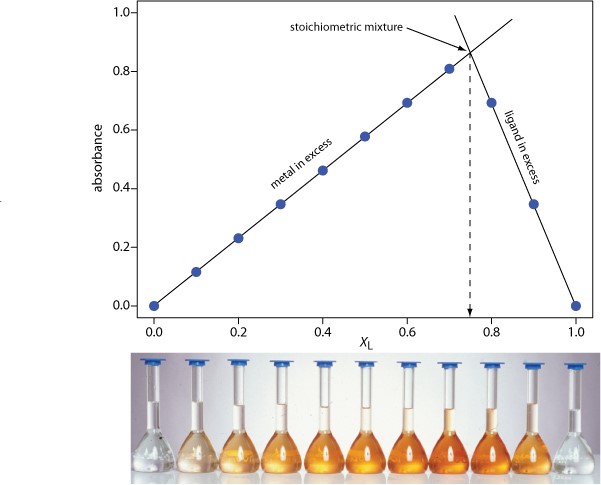
8.2: Formation Constants Lab - Chemistry LibreTexts

KR20190042056A - Composition and method thereof - Google Patents
8.2: Formation Constants Lab - Chemistry LibreTexts
Why do we have to assume that the base is weak if we add a strong
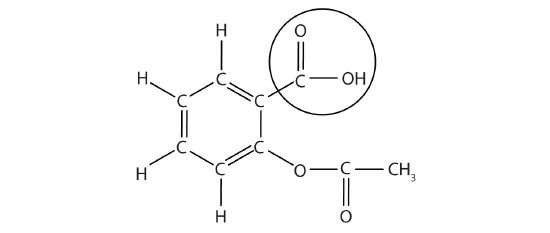
10.5: Buffers - Chemistry LibreTexts
14.5: Polyprotic Acids - Chemistry LibreTexts

14.3: Relative Strengths of Acids and Bases - Chemistry LibreTexts

Recrystallization Of Organic Chemistry Lab Report

14.6: Buffers - Chemistry LibreTexts